Original Research
Surgical Excision versus Ultrasound-Guided Treatment of Symptomatic Wrist Ganglia in Pediatric Patients
1Department of Orthopedic Surgery, University of Massachusetts, Worcester, MA; 2Edward B. Singleton Department of Pediatric Radiology, Texas Children’s Hospital, Houston, TX; 3Department of Orthopedic Surgery, Baylor College of Medicine, Houston, TX; 4Department of Pediatric Radiology, Johns Hopkins All Children’s Hospital, St. Petersburg, FL; 5Department of Pediatric Orthopedic Surgery, Texas Children’s Hospital, Houston, TX
Correspondence: Bryce Bell, MD, Department of Pediatric Orthopedic Surgery, Texas Children’s Hospital, 18200 W. Katy Freeway, 5th floor, Houston, TX 77094. E-mail: [email protected]
Received: June 5, 2022; Accepted: December 27, 2022; Published: February 1, 2023
Volume 5, Number 1, February 2023
Abstract
Background: Multiple invasive treatments exist for pediatric patients presenting for evaluation of symptomatic wrist ganglia, most commonly surgical excision or percutaneous treatment. Treatment modality is often dictated by patient preference and recurrence rate. Compared with adult literature, treatment outcomes of wrist ganglia in children are less examined. This study examines recurrence rates of wrist ganglia after surgical excision and ultrasound-guided treatment (USGT) in a pediatric population.
Methods: A retrospective study of patients referred for wrist ganglia treatment from January 2015 to February 2020 at a single-center children’s hospital was performed. Patients aged 0-18 years with a minimum of 1-year follow-up from either USGT or surgical excision were included.
Results: Sixty-nine patients underwent surgical excision with 15 (22%) experiencing recurrence. Seventy-seven patients underwent USGT with 26 (34%) experiencing recurrence. There was no statistical difference between recurrence rates between the two groups (p = 0.140). Thirty of 146 (20.5%) patients underwent a subsequent excision procedure where 18 (60%) experienced no recurrence, 4 (13%) experienced recurrence, and 8 (26%) had insufficient follow-up of less than one year. All 7 patients treated in clinic with aspiration without ultrasound guidance required subsequent intervention.
Conclusion: USGT of wrist ganglia is a reasonable treatment choice for pediatric patients and has a similar recurrence rate to surgical excision. For patients that require subsequent treatment, recurrence rates are also similar between USGT and surgical excision. Parents of children with wrist ganglia should be educated about treatment options and recurrence rates, and patients should have age-appropriate involvement in management decisions regarding treatment of wrist ganglia.
Level of Evidence: Level III
Key Concepts
- Surgical excision and USGT of wrist ganglia have similar recurrence rates in the pediatric population.
- Recurrence rates range from 22-33% in patients after a single procedure.
- There is a low rate of recurrence when recurrent ganglia are treated with surgical excision.
Introduction
Ganglia account for 50–70% of all soft-tissue masses presenting in the hand and wrist.1 These myxoid lesions are made up of gelatinous, thick mucin connected to a joint or tendon sheath by a stalk, built of organized collagen fibers.2 While often asymptomatic, some patients complain of pain, tenderness, and cosmetic concerns.1 There are multiple treatment options for ganglia; however, the most common complication amongst all modalities is ganglion recurrence with rates as high as 87% depending on methodology.3 The most common invasive treatments of symptomatic ganglia are aspiration, surgical excision, and arthroscopic resection. Studies examining recurrence rates among adults with ganglia have sought to quantify recurrence rates after procedures in hopes of guiding treatment choice. Published recurrence rates vary widely from 1-34%,2,4–9 5-77%,10–16 and 3-20%17–21 after excision, aspiration, and arthroscopy, respectively.
While there is a great deal of research on ganglia in the adult population, there is a relative paucity of data in pediatric patients. There are differences in both patient and ganglion characteristics in pediatric populations. Pediatric patients have a higher incidence of tendon sheath ganglia compared to adults,22 and although 48%24 to 66%25 of pediatric ganglia resolve with observation alone, concurrent splinting can increase resolution rates up to 79%.22 Yet, invasive treatment is indicated for recurrent, painful ganglia, and those that do not self-resolve.26 Recent literature reports higher recurrence rates in previously treated pediatric ganglia as compared to treated adults,22,23,27 with published pediatric surgical recurrence ranging from 2.8%-35%.23,28,29 As ganglia recurrence after invasive treatment plays an important factor in guiding clinical practice and shared decision-making with families, we sought to compare the recurrence rates between surgical excision and ultrasound-guided treatment (USGT) of pediatric wrist ganglia. We hypothesized that surgical excision would result in significantly lower recurrence rates than USGT of pediatric wrist ganglia.
Materials and Methods
A retrospective cohort study to compare and quantify recurrence rates between surgical excision and USGT of pediatric wrist ganglia was conducted from a free-standing tertiary referral academic children’s hospital following approval by the Institutional Review Board. Patients with an ICD-9 or ICD-10 code of a ganglion cyst of the wrist and who underwent invasive treatment for this diagnosis were queried from January 2015 to February 2020 to identify all potential subjects. All patients aged 0-18 years with a minimum of 1-year follow-up from the original procedure were included in the study. Clinic notes were then manually examined to ensure that each patient was presenting for treatment of a symptomatic ganglia of the wrist. Patients were excluded for those with less than 1-year asymptomatic follow-up and/or those who presented for treatment of hand or forearm ganglia (rather than wrist).
Surgical excision was performed by a fellowship trained pediatric hand surgeon. Non-surgical treatment was performed in two settings: in clinic by a pediatric hand surgeon without image guidance or USGT by a fellowship-trained pediatric musculoskeletal radiologist. Subgroup analysis was delineated based upon treatment methodology. USGT followed previously published protocols including aspiration and fenestration with instillation of steroid and local anesthetic followed by compression and brace wear.12,30
Patient demographics and recurrence were collected via chart review. Clinic notes and/or post-procedural calls were utilized to verify recurrence, defined as a palpable mass in the same location as the original complaint.
Statistical Analysis
Descriptive statistics were utilized to analyze differences in recurrence rates for each cohort. Student’s t-test was used to determine differences in continuous variables. Chi-square was used to determine differences in categorical variables. Where the assumptive conditions of the chi-square test were not met, Fisher’s exact test was used to determine statistical differences. Backwards, stepwise binary logistic regression was used to control for confounding variables and identify risk factors for ganglia recurrence. Predictor variables included age (years), gender (male referent), and location (dorsal referent). Odds ratios (OR) and 95% confidence intervals were calculated and recorded for each regression variable. Alpha was set to a significance level of 0.05 for all analyses. Statistical analyses were performed with R software MASS version 7.3.51.6 (www.r-project.org).
An a priori power analysis was conducted using IBM SPSS Statistics V.27.0 (IBM Corp., Armonk, NY) for sample size estimation, based on the data from “Treatment of Ganglion Cysts” by Suen et al. in 2013 (Naspiration = 191, Nsurgery = 884), a systematic review of wrist ganglia recurrence rates after aspiration and surgical excision.31 The effect size in the aforementioned study was calculated to be 2.31, considered to be extremely large using Cohen’s 1988 criteria.32 With a significance criterion of α = 0.05 and power = 0.95, the minimum total sample size needed with this effect size is n = 60 for independent t-test. Thus, the obtained sample size of 146, 77 in the USGT cohort and 69 in the surgical cohort, was deemed more than adequate to test the study hypothesis.
Results
One hundred fifty-three total patients were identified. Sixty-nine (45.1%) patients underwent surgical excision, 77 (50.3%) patients underwent USGT, and 7 (4.6%) patients underwent treatment in clinic with aspiration without ultrasound assistance. All patients had a mean age of 12.3 ± 3.9 years old, 10.9 ± 4.2 in the surgical excision cohort and 13.5 ± 3.2 in the USGT cohort. There was a significant different between the ages of the cohorts (p < 0.001). The female to male ratio was 2.4:1 between cohorts (Table 1).
Table 1. Demographics of Patients Grouped by Initial Procedure
| Initial procedure | p-value | ||
|---|---|---|---|
| Excision | USGT | ||
| Total patients | 69 | 77 | |
| Age (mean ± SD) | 10.9 ± 4.2 | 13.5 ± 3.2 | <0.001* |
| Female | 48 | 54 | 1 |
| Male | 21 | 23 | |
| Female:Male | 2.3 | 2.3 | |
| Dorsal | 43 | 57 | 0.154 |
| Volar | 26 | 20 | |
| Dorsal:Volar | 1.7 | 2.9 | |
| Recurrence | 15 | 26 | 0.14 |
| No Recurrence | 54 | 51 | |
| Recurrence rate | 22% | 34% | |
Key: SD: standard deviation, *: significant
One hundred percent of patients who underwent treatment in-clinic with aspiration without the aid of ultrasound experienced recurrence. Six of these patients (85.7%) were subsequently treated with surgical excision and one (14.3%) via USGT. No additional recurrences were documented within the 1-year follow-up period.
In the surgical cohort, 15 (22%) patients experienced recurrence, while in the USGT cohort, 26 (34%) patients experienced recurrence. The difference between the two groups did not reach statistical significance (p=0.14). Regression analysis of recurrence risk factors showed that age (OR = 1.048, 95%CI [0.945, 1.162], p=0.43), gender (OR = 0.873, 95%CI [0.396, 1.979], p=0.68), and ganglion location (OR = 0.895, 95%CI [0.385, 2.001], p=0.86) did not confer an increased risk of recurrence (Table 2).
Table 2. Patient Characteristics Grouped by Recurrence after Initial Procedure
| Recurrence | OR (95% CI) | ||
|---|---|---|---|
| Yes | No | ||
| Total patients | 41 | 105 | |
| Age (mean ± SD) | 12.3 ± 4.0 | 12.2 ± 3.5 | 1.048 (0.945 - 1.162) |
| Male | 13 | 31 | 0.873 (0.396 - 1.979) |
| Female | 28 | 74 | |
| Female:Male | 2.2 | 2.4 | |
| Dorsal | 17 | 49 | 0.895 (0.385 - 2.001) |
| Volar | 12 | 34 | |
| Dorsal:Volar | 1.4 | 1.4 | |
| Excision | 15 | 54 | 2.064 (0.939 - 4.715) |
| USGT | 26 | 51 | |
Treatment for recurrent ganglia in both cohorts (n = 41) varied widely. Eleven (26.8%) patients elected observation, 24 (58.5%) elected surgical excision, 3 (7.3%) elected repeat USGT, and 3 (7.3%) elected in office treatment (Figure 1). Of the 30 patients (20.5%) who underwent a subsequent procedure, 18 (60%) experienced no symptomatic recurrence, 4 (13%) experienced recurrence, and 8 (26%) had insufficient follow-up of less than 1 year from their repeat procedure at the time of data collection. There was no statistically significant difference in recurrence rates based upon subsequent procedure (p=0.29).
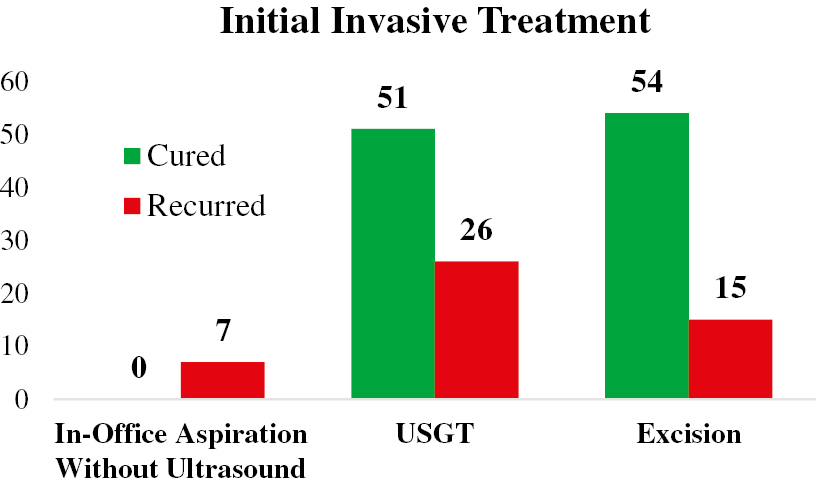
Figure 1. Clinical course of patients treated for wrist ganglia subdivided by initial treatment, recurrence, and subsequent procedures. USGT: Ultrasound-guided treatment.

Discussion
There are various invasive treatment options available for symptomatic wrist ganglia, including USGT, in-office aspiration without the aid of imaging, and surgical excision. The main complication of symptomatic wrist ganglia, regardless of treatment, is recurrence. Our results showed no statistically significant difference in symptomatic recurrence rates between USGT and surgical excision for invasive treatment of pediatric wrist ganglia.
Historically, patients underwent unguided aspiration in clinic and in our cohort, all seven ganglia treated in clinic eventually recurred. However, recent studies have described more detailed and accurate methods for outpatient treatment of wrist ganglia. At our institution, ultrasound guidance is utilized for more complete treatment of ganglia, employing well-defined protocols yielding a 29% recurrence rate.30 USGT offers several advantages in pediatric populations. With image guidance, the needle tip can be directly visualized within the lesion leading to confirmation of ganglion collapse and non-refilling. Additionally, damage to local structures can be avoided. Previous research conducted demonstrated that USGT was well tolerated, led to no complications, and required no general sedation in the pediatric population.30
USGT recurrence rate of 34% was far lower than previously reported aspiration recurrence rates, which have reached as high as 82% in previous literature.27 Additionally, a recent multi-center study reported a recurrence rate of 27% with surgical excision compared to 82% with aspiration, although aspiration methodology was not reported.34 Reported recurrence rates after surgical excision are similar to our analysis, yet the rate of recurrence after aspiration is significantly lower in our study.34 The authors attribute the success of USGT to improved visualization and complete aspiration of ganglia by a trained musculoskeletal radiologist, in addition to protocolized fenestration, compression, and brace wear.12,35
Although surgical excision had a lower rate of recurrence than USGT, percutaneous treatment affords multiple benefits. The great majority can be accomplished with local anesthetic, removing the need for and risks of general anesthesia. The duration of the procedure is shorter, and there is minimal scar tissue formation. Although our project did not examine functional changes to wrist movement after procedures, Angelides et al. reported 1.2% of patients experienced 0-10 degree loss of flexion after surgery.4 Others have reported residual pain36 and loss of grip strength37 after ganglia excision.
At our institution, surgical excision is performed under general anesthesia. Although adult patients can undergo surgical excision of ganglia under regional or local anesthesia, excision was felt to be better tolerated under general anesthesia for the pediatric population while also minimizing possible complication from patient movement during the procedure. In addition, USGT is substantially less expensive than surgical excision as separate charges for operating space, anesthesiology, and recovery are all avoided.
In recurrent ganglia, 30 patients pursued subsequent treatment, with 18% (4/22) of patients with adequate follow-up experiencing additional recurrence. Subsequent treatment with surgical excision was particularly successful. Fifteen of 16 patients experienced resolution of ganglia (6.25% recurrence rate). Compared to the rate of recurrence in primary surgical excision (23%), lower recurrence in subsequent treatment with surgical excision may be explained by a tendency towards more aggressive excision in recurrent ganglia.
There was a significant difference in the ages of patients between the two treatment groups, with younger patients more frequently undergoing surgical excision. Surgical excision is done under general anesthesia and may thus be a more attractive treatment for young patients who are more prone to anxiety with awake USGT. While there was no formal criteria for recommending either procedure on the basis of age and there was no minimum age criteria that must be met for USGT, age may have played a role in the decision-making process shared between physicians and the patients’ families.
Several characteristics have been identified in the pediatric population that suggest a risk for recurrence of ganglia. In our cohort, age, gender, and ganglia location did not confer an increased risk for recurrence. Similarly, Mooney et al. examined common characteristics in pediatric recurrent ganglia, reporting no statistical difference in recurrence rates between dorsal and volar ganglia.35 Conversely, Mooney et al. reported that recurrence was more frequent in older patients,35 which was not supported by our analysis. Female prevalence of wrist ganglia is well established in pediatric populations; we report a female to male ratio of 2.4:1, supported by previous literature with gender ratios ranging from 1.6:127 to 4.7:1.22
While these results can inform the decision to treat symptomatic ganglia in pediatric patients, it should be noted that the first line treatment for patients with wrist ganglia at our institution was observation with or without bracing for mildly symptomatic or asymptomatic ganglia. Invasive procedures were only considered if the patient 1) had failed conservative management for >6 months or 2) was complaining of intense pain that adversely affected daily activities or sports participation. Patients not meeting these criteria were given regular follow-up to ensure resolution of symptoms.
This analysis was a single-center retrospective cohort, and therefore, subject to inherent biases and confounders including regional bias, information bias, and systematic bias. For example, the surgical data was procured through chart review, which may fail to capture ganglion recurrence that is painless, contributing to both information bias and systematic bias. Moreover, patients in the USGT cohort were reached by telephone which is subject to response bias. Additionally, dorsal ganglia more often treated with USGT compared to volar ganglia which may influence risk factor sub-analysis.
Conclusion
In pediatric patients with symptomatic wrist ganglia, patients and families that desire intervention should be counseled on the similar symptomatic recurrence rates between USGT and surgical excision. For recurrent ganglia, patients can be subsequently treated by either method with low likelihood of recurrence. Families should be engaged in the decision-making process with the physician to determine the optimal procedure for their child.
Additional Links
- POSNAcademy: Pediatric Benign Soft Tissue Tumors, Christine A. Ho, MD
- Journal of Medical Insight: Pediatric Excision of Ganglion Cyst from Right Wrist, Marcus Lester R. Suntay, MD, FPCS, FPSPS, FPALES
Disclaimer
No funding was received. The authors have no conflicts of interest to report.
References
- Minotti P, Taras JS. Ganglion cysts of the wrist. J Hand Surg. 2002;2(2):102–107.
- Kuliński S, Gutkowska O, Mizia S, et al. Dorsal and volar wrist ganglions: the results of surgical treatment. Adv Clin Exp Med. 2019;28(1):95-102.
- Rosson JW, Walker G. The natural history of ganglia in children. J Bone Joint Surg Br. 1989;71:707-708.
- Angelides AC, Wallace PF. The dorsal ganglion of the wrist: its pathogenesis, gross and microscopic anatomy, and surgical treatment. J Hand Surg Am. 1976;1(3):228-235.
- Zachariae L, Vibe-Hansen H. Ganglia. Recurrence rate elucidated by a follow-up of 347 operated cases. Acta Chir Scand. 1973;139(7):625-628.
- Singhal R, Angmo N, Gupta S, et al. Ganglion cysts of the wrist: a prospective study of a simple outpatient management. Acta Orthop Belg. 2005;71(5):528-534.
- Craik JD, Walsh SP. Patient outcomes following wrist ganglion excision surgery. J Hand Surg Eur Vol. 2012;37(7):673-677.
- Gündeş H, Cirpici Y, Sarlak A, et al. Prognosis of wrist ganglion operations. Acta Orthop Belg. 2000;66(4):363-367.
- Faithfull DK, Seeto BG. The simple wrist ganglion--more than a minor surgical procedure? Hand Surg. 2000;5(2):139-143.
- Richman JA, Gelberman RH, Engber WD, et al. Ganglions of the wrist and digits: results of treatment by aspiration and cyst wall puncture. J Hand Surg Am. 1987;12(6):1041-1043.
- Zubowicz VN, Ishii CH. Management of ganglion cysts of the hand by simple aspiration. J Hand Surg Am. 1987;12(4):618-620.
- Zeidenberg J, Aronowitz JG, Landy DC, et al. Ultrasound-guided aspiration of wrist ganglions: a follow-up survey of patient satisfaction and outcomes. Acta radiol. 2016;57(4):481-486.
- Gitto S, Lee SC, Miller TT. Ultrasound-guided percutaneous treatment of volar radiocarpal ganglion cysts: safety and efficacy. J Clin Ultrasound. 2019;47(6):339-344.
- Wright AA, Taylor JB, Ford KR, et al. Risk factors associated with lower extremity stress fractures in runners: a systematic review with meta-analysis. Br J Sports Med. 2015;49(23):1517-1523.
- Otu AA. Wrist and hand ganglion treatment with hyaluronidase injection and fine needle aspiration: a tropical African perspective. J R Coll Surg Edinb. 1992;37(6):405-407.
- Jagers Op Akkerhuis M, Van Der Heijden M, Brink PRG. Hyaluronidase versus surgical excision of ganglia: a prospective, randomized clinical trial. J Hand Surg Br. 2002;27(3):256-258.
- Fernandes CH, Miranda CDO, Dos Santos JBG, et al. A systematic review of complications and recurrence rate of arthroscopic resection of volar wrist ganglion. Hand Surg. 2014;19(3):475-480.
- Kang L, Akelman E, Weiss APC. Arthroscopic versus open dorsal ganglion excision: a prospective, randomized comparison of rates of recurrence and of residual pain. J Hand Surg Am. 2008;33(4):471-475.
- Rizzo M, Berger RA, Steinmann SP, et al. Arthroscopic resection in the management of dorsal wrist ganglions: results with a minimum 2-year follow-up period. J Hand Surg Am. 2004;29(1):59-62.
- Gallego S, Mathoulin C. Arthroscopic resection of dorsal wrist ganglia: 114 cases with minimum follow-up of 2 years. Arthroscopy. 2010;26(12):1675-1682.
- Fernandes CH, Meirelles LM, Raduan Neto J, et al. Arthroscopic resection of dorsal wrist ganglion: results and rate of recurrence over a minimum follow-up of 4 years. Hand (N Y). 2019;14(2):236-241.
- Wang AA, Hutchinson DT. Longitudinal observation of pediatric hand and wrist ganglia. J Hand Surg Am. 2001;26(4):599-602.
- Satku K, Ganesh B. Ganglia in children. J Pediatr Orthop. 1985;5(1):13-15.
- Zinger G, Michailevich M, Bregman A, et al. Wrist ganglia in children: nonsurgical versus surgical treatment. J Hand Surg Am. 2020;45(6):551.e1-551.e5.
- Petricig P, Pepe E. [First choice treatment of the pediatric wrist ganglia]. Minerva Pediatr. 2006;58(4):379-383.
- Meyerson J, Pan YL, Spaeth M, et al. Pediatric ganglion cysts: a retrospective review. Hand (N Y). 2019;14(4):445-448.
- Mackinnon AE, Azmy A. Active treatment of ganglia in children. Postgrad Med J. 1977;53(621):378-381.
- Colon F, Upton J. Pediatric hand tumors. A review of 349 cases. Hand Clin. 1995;11(2):223-243.
- Simon Cypel TK, Mrad A, Somers G, et al. Ganglion cyst in children: reviewing treatment and recurrence rates. Can J Plast Surg. 2011;19(2):53-55.
- Schallert EK, Cano MC, Ditzler MG, et al. Percutaneous ultrasound-guided ganglion fenestration in children: initial results. Skeletal Radiol. 2021;50:1169-1175.
- Suen M, Fung B, Lung CP. Treatment of ganglion cysts. ISRN Orthop. 2013;2013:940615.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Routledge Academic; 1988.
- Colberg RE, Sánchez CF, Lugo-Vicente H. Aspiration and triamcinolone acetonide injection of wrist synovial cysts in children. J Pediatr Surg. 2008;43(11):2087-2090.
- Shanks C, Schaeffer T, Falk DP, et al. The efficacy of nonsurgical and surgical interventions in the treatment of pediatric wrist ganglion cysts. J Hand Surg Am. 2022;47(4):341-347.
- Mooney ML, Jacobs CA, Prusick VW, et al. Pediatric ganglion cyst recurrence: location isn’t the only risk factor. J Pediatr Orthop. 2020;40:340-343.
- Sanders WE. The occult dorsal carpal ganglion. J Hand Surg Br. 1985;10(2):257-260.
- Dias JJ, Dhukaram V, Kumar P. The natural history of untreated dorsal wrist ganglia and patient reported outcome 6 years after intervention. J Hand Surg Eur Vol. 2007;32(5):502-508.
